Sodium-Glucose Cotransporter-2 (SGLT-2) Inhibitors in Patients with Diabetes and Gout Comorbidity
Arzu Patel, PharmD Candidate;
Cortney Mospan, PharmD, BCACP, BCGP, CPP, Assistant Professor of Pharmacy
Wingate University School of Pharmacy
As of 2018, nearly 34.2 million Americans (10.5% of the population) are living with diabetes. Individuals with diabetes are at increased risk of developing cardiovascular disease due to comorbidities, such as hypertension and dyslipidemia.1 These comorbid conditions in conjunction with damage caused by sustained hyperglycemia can lead to damage to vasculature.2 As a result, patients with diabetes have a two-fold increased risk of mortality due to heart disease and stroke.3
Type 2 diabetes is also often associated with chronically elevated serum uric acid levels, which can further elevate risk of hypertension, cardiovascular disease, and chronic kidney disease.4 Since the 1950s, gout has been recognized to be associated with diabetes and common complications of diabetes (e.g., cardiovascular disease, hypertension, kidney disease).5 Elevated uric acid levels associated with gout further increase the risk of mortality in patients with diabetes as every 1 mg/dL increases the risk of all-cause death by 39%.6
Although there are several mechanisms by which serum uric acid levels can become elevated, in the context of type 2 diabetes, it is often correlated with excessive dietary fructose. This results in extensive phosphorylation of fructose by fructokinase in the liver, depleting its ATP stores, and resulting in the conversion of adenosine into uric acid.4Further, many foods that patients with diabetes may select (e.g., seafood, diet soft drinks) have been associated with an increased risk of hyperuricemia and gout.7 Nearly 25% of individuals with type 2 diabetes also have gout.8
The American Diabetes Association (ADA) 2020 Standards of Care recommend a patient-centered approach to guide the selection of pharmacologic agents for management of diabetes. Such factors that are recommended for consideration include cost, side effects, patient preferences, weight impact, risk of hypoglycemia, and presence of cardiovascular comorbidities. Patients with established atherosclerotic cardiovascular disease (ASCVD) or who have high risk of development of ASCVD, heart failure, or kidney disease are recommended to be prescribed either an SGLT-2 inhibitor or glucagon-like peptide-1 (GLP-1) receptor agonist with demonstrated cardiovascular benefit.10Table 1 provides a summary of expected A1c lowering and cardiovascular indications for SGLT-2 inhibitors and GLP-1 receptor agonists that are currently on the market.
Table 1. Available SGLT-2 Inhibitors and GLP-1 Receptor Agonists11-17
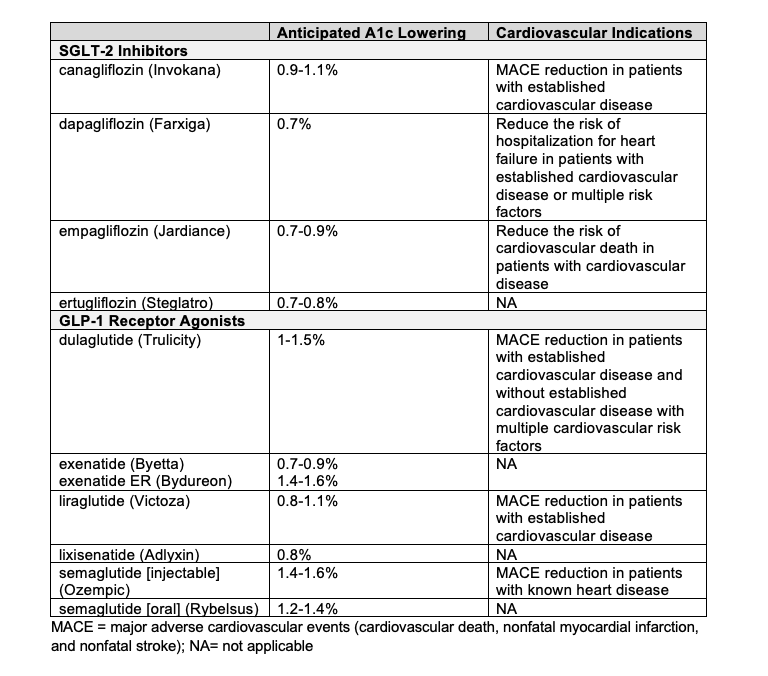
SGLT-2 inhibitors have been found to reduce risk of cardiovascular and renal events by affecting hemodynamic changes and body weight reduction, as well as protecting myocardial, endothelial, and tubulo-glomerular functions and reducing glucotoxicity. SGLT-2 inhibitors may also contribute to the lowering of serum uric acid levels. Although the exact mechanism is unknown, it is postulated that this is related to its induction of renal glucose elimination.4 In promoting glucosuria, these medications may also lead to the suppression of glucose transporter 9b (GLUT9b), which is a bexose/urate transporter located at the proximal tubular cells across the basolateral membrane.18 As a result, there is also promotion of uric acid excretion, resulting in improved cardiovascular and renal function and decreased risk of gouty arthritis, renal tubulo-interstitial fibrosis, and chronic kidney disease.4
A meta-analysis of 62 clinical trials involving SGLT-2 inhibitors was conducted with a total patient population of 34,941 patients. Patients with diabetes who were treated with an SGLT-2 inhibitor had reductions from baseline serum uric acid concentrations by 0.59-0.75 mg/dL.4 This could provide potentially clinically relevant uric acid lowering, as Maravic et al. found that just 39% of patients with gout reached their uric acid goal of < 6 mg/dL.19These effects were observed within days of initiating treatment and were persistent after two years. It was also observed that patients with higher hemoglobin A1c experienced greater reductions in serum uric acid concentrations, which is consistent with greater uricosuria accompanying greater glucouria. This was considered a class effect, due to inconsistent data amongst studies and numerous confounders.4 Table 2 provides a summary of uric acid lowering of SGLT-2 inhibitors. No studies have evaluated the uric acid lowering potential of ertugliflozin specifically, so this SGLT-2 inhibitor would not be preferred in patients with diabetes and gout comorbidity. While The uric acid lowering is greatest with empagliflozin, differences are fairly minor between studied SGLT-2 inhibitors, thus, uric acid reduction differences should not guide which SGLT-2 inhibitor is selected. This decision should be based on A1c lowering, other cardiovascular indications and coverage by insurance.
Table 2. Uric Acid Lowering of SGLT-2 inhibitors

In the EMPA-REG OUTCOME trial, it was found that patients taking empagliflozin had lower serum uric acid concentrations than those receiving placebo. After one year of treatment, risk of gout was decreased by 73%.20Fralick et al. recently published a population-based cohort study that evaluated the rate of new onset gout in patients (N=295,907) with diabetes prescribed an SGLT-2 inhibitor compared to those prescribed a GLP-1 receptor agonist. Patients were not included in the analysis if they had a diagnosis of gout or had previously received gout therapy. Patients prescribed an SGLT-2 inhibitor had a lower rate of new onset gout with 4.9 events per 1000 person-years compared to 7.8 events per 1000 person-years. This resulted in a hazard ratio of 0.64 (95% confidence interval 0.57-0.72). The authors concluded that SGLT-2 inhibitors may decrease the risk of gout, but further studies are necessary to confirm this observation. Limitations of this study include lack of measurement of or accounting for confounding, a low baseine risk of gout in the study population, the short length of study (~9 months) and missing or incomplete data due to the retrospective nature of the study. 21 Of note, two other commonly utilized diabetes medications, GLP-1 receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors have also been evaluated in gout comorbidity and appear to have little to no effect on serum uric acid concentrations.18
Currently, there are limited medications available to manage hyperuricemia associated with gout, and many patients are sub-optimally managed. Febuxostat may be associated with higher cardiovascular and all-cause mortality risk when compared to allopurinol, which is now recommended as first-line urate lowering therapy for patients with gout.22 While their effects on uric acid levels still need further investigation, SGLT-2 inhibitors do appear to offer a fair amount of uric acid lowering and may be a preferred glucose lowering treatment in patients with diabetes and gout comorbidity. There is some possibility of increased risk of renal calculi; however, there are currently no studies available to evaluate possible long-term risk associated with its use.18 Use of SGLT-2 inhibitors in patients with a history of kidney stones caused by uric acid formation should be carefully considered given this potential risk of use. Based on the cardiovascular risk reduction offered by SGLT-2 inhibitors, coupled with reduction in serum uric acid concentrations, these medications may be useful as an additive treatment in patients with both gout and type 2 diabetes.4
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020.
- Diabetes, Heart Disease, and Stroke [Internet]. National Institute of Diabetes and Digestive and Kidney Diseases. U.S. Department of Health and Human Services; 2017 [cited 2020Apr6]. Available from: https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/heart-disease-stroke
- Tung YC, Lee SS, Tsai WC, Lin GT, Chang HW, Tu HP. Association between gout and incident type 2 diabetes mellitus: a retrospective cohort study. Am J Med. 2016;129(11):1219.e17-1219.e25.
- Bailey CJ. Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes Obes Metab. 2019;21:1291-128.
- Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811-1821.
- Ioachimescu AG, Brennan DM, Hoar DM, et al. Serum uric acid is an independent predictor of all-cause mortality in patients at high risk of cardiovascular disease: A preventive cardiology information system (PreCIS) database cohort study. Arthritis Rheum. 2008;58:623-630.
- Roddy E, Choi HK. Epidemiology of gout. Rheum Dis Clin North Am. 2014;40:155-175.
- Tung Y, Lee S, Tsai W, et al. Association between Gout and Incident Type 2 Diabetes Mellitus: A Retrospective Cohort Study. Am J Med. 2016;129(11):1219.e17-1219.e25.
- American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care – 2020. Diabetes Care. 2020;43(Suppl 1):S98-S110.
- American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care – 2020. Diabetes Care. 2020;43(Suppl 1):S111-134.
- Gillenwater BA, Wakefield AN, Triboletti J, Gonzalvo JA, Meredith AH. GLP-101: A diabetes educator’s guide to glucagon-like-peptide-1 receptor agonists. AADE In Practice. 2019;7(5):32-41.
- Pearson S, Kietsiriroje N, Ajjan RA. Oral semaglutide in the management of Type 2 Diabetes: A report on the evidence to date. Diabetes Metab Syndr Obes. 2019;12:2515-2529.
- Dagogo-Jack S, Liu J, Eldor R, et al. Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: the VERTIS SITA2 placebo-controlled randomized study. Diabetes Obes Metab. 2018;20(3):530–540.
- Triplett C, Cornell S. Canagliflozin treatment in patients with type 2 diabetes mellitus. Clin Med Insights Endocrinol Diabetes. 2015;8:73-81.
- Zimmerman J. Empagliflozin (Jardiance) for type 2 diabetes mellitus. Am Fam Physician. 2016;94(12):1014-1015.
- Pong CK. Dapagliflozin (Farxiga) for type 2 diabetes mellitus. Am Fam Physician. 2015;91(12):828-833.
- Lexicomp Online Database [database on the Internet]. Hudson (OH): Lexicomp Inc.: 2020 [cited 15 May 2020].
- Sheu WHH. Lowering the risk of gout: Another benefits from the use of sodium-glucose cotransporter 2 inhibitors. J Diabetes Investig. 2020; in press. doi: 10.1111/jdi.13254.
- Maravic M, Hincapie N, Pilet S, et al. Persistent clinical inertia in gout in 2014: An observational French longitudinal patient database study. Joint Bone Spine. 2018;85:311-315.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
- Fralick M, Chen SK, Patorno E, Kim SC. Assessing the risk for gout with sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes. Ann Intern Med. 2020;172:186-194.
- Fitzgerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Rheumatol. 2020 Jun;72(6):879-895.