Euglycemic Diabetic Ketoacidosis and Sodium Glucose Cotransporter-2 Inhibitors
Haleigh Stolte, PharmD
PGY1 Pharmacy Practice Resident, North Kansas City Hospital
Kathryn Holt, PharmD, BCPS
Clinical Assistant Professor, UMKC School of Pharmacy
Sodium glucose cotransporter-2 (SGLT2) inhibitors are an effective class of antidiabetic medications commonly prescribed for patients with type II diabetes. They are available as single agents (canagliflozin, dapagliflozin, empagliflozin, ertugliflozin) or in combination products with metformin. SGLT2 inhibitors work by blocking reabsorption of glucose in the proximal renal tubules, leading to increased excretion through the urine. SGLT2 inhibitors have gained popularity after clinical trials showed benefit in reducing cardiovascular events in patients with type II diabetes.1,2 However, these medications do carry warnings for urinary tract infections, lower limb amputations, and acute renal failure.3 In 2015, the U.S. Food and Drug Administration (FDA) issued an additional warning regarding the potential risk of acidosis and ketoacidosis in the setting of near normal blood glucose levels.4 This warning came to light after 73 clinical cases of euglycemic diabetic ketoacidosis (DKA) were reported in patients taking an SGLT2 inhibitor in a 15 month period.4
Diabetic ketoacidosis (DKA) is a serious acute complication of diabetes which can occur in patients with type I or type II diabetes. Symptoms including polyuria, polydipsia, nausea, and vomiting; or in severe cases, Kussmaul respirations, altered mental status, and coma. DKA is defined by the by three cardinal laboratory markers: hyperglycemia, metabolic acidosis, and increased plasma ketones. In rare cases, DKA occurs in the absence of hyperglycemia, referred to as euglycemic DKA, which is defined by the American Diabetes Association (ADA) as DKA despite a blood glucose < 250 mg/dL.5-7
Euglycemic DKA was first identified by Munro, et al in a 1973 case series which analyzed 211 cases of DKA. Of these cases, 37 occurrences of DKA had near normal blood glucose values, despite other symptoms of DKA. The patients in this case series were primarily patients with type I diabetes who had recent vomiting, carbohydrate reductions, or alterations in their insulin doses.8 Treatment of euglycemic DKA mimics the treatment of traditional cases of DKA. Intravenous fluids, dextrose, potassium supplementation, and continuous insulin infusions should be started as soon as clinically feasible. The difference between treatment modalities with euglycemic DKA is that dextrose may need to be initiated sooner for patients in euglycemic DKA due to lower blood glucose levels.5
Several case reports have been published describing euglycemic DKA in type II diabetics on SGLT2 inhibitors.7, 9-12 In these reports, patients often present with previous illness or postoperatively, in which results in carbohydrate reduction. Frequently, other causes of metabolic acidosis are ruled out before treatment for euglycemic DKA is initiated. When treatment begins, a patient’s anion gap metabolic acidosis resolves. In cases of patients who develop euglycemic DKA after surgical procedures, holding SGLT2 inhibitors has not been recommended because the duration of metabolic effects is unknown.7
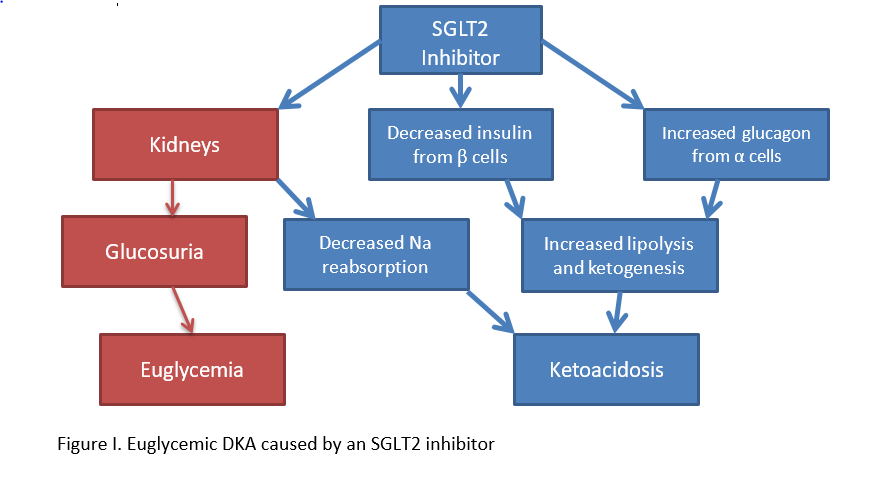
Several mechanisms for euglycemic DKA have been hypothesized, including starvation- induced, pregnancy-associated, alcohol-associated, and SGLT-2-induced. Euglycemia caused by starvation, pregnancy, or alcohol intake results from a lack of caloric intake and increased production of counter regulatory hormones that lead to urinary excretion of glucose, thus resulting in near normal blood glucose levels. Euglycemic DKA caused by an SGLT2 inhibitor is detailed in Figure I. SGLT2 inhibitors act on beta and alpha cells, which can result in ketoacidosis. Furthermore, as with other proposed mechanisms of euglycemic DKA, SGLT2 inhibitors cause an increase in urine loss glucose, resulting in normal blood glucose levels in the presence of acidosis.9-12
The FDA Adverse Event Reporting System (FAERS) is a database of adverse drug events, medication error reports, or product quality issues to which healthcare professionals can report.. A review of this public database reveals that in 2018, 174 cases of euglycemic DKA were reported to the FDA, of which approximately 83% were due to concomitant use of a SGLT2 inhibitor. Factors which may trigger the onset of euglycemic DKA are similar to hypothesized mechanisms stated previously, and include current illness, reduced fluid/caloric intake, insulin dose alterations, and/or alcohol intake.4
The primary concern surrounding euglycemic DKA is missed diagnosis. As a rare side effect where patients present without the cardinal symptoms of DKA, clinicians may look for for other causes of metabolic acidosis before proper treatment is initiated. In order to avoid missing clinical cases of euglycemic DKA, a thorough medication history and awareness of the warning associated with SGLT2 inhibitors is key. Additionally, patients prescribed SGLT2 inhibitors may be educated to check their urine ketones, increase hydration, and avoid reducing carbohydrate intake during sick days.13
In summary, SGLT2 inhibitors are an effective class of antidiabetic medications with cardiovascular benefits; however, euglycemic DKA can occur in patients taking SGLT2 inhibitors. Patients present with anion gap acidosis, serum/urine ketones, and blood glucose values less than 250 mg/dL. Once identified, treatment is similar to DKA treatment, although dextrose-containing fluids may need to be initiated sooner to prevent hypoglycemia. Serum and/or urine ketones may be monitored when patients on SGLT2 inhibitors are hospitalized or ill. Further research must be done before recommendations on holding SGLT2 inhibitors prior to surgery can be made. Any cases of euglycemic DKA that occurs in patients on an SGLT2 inhibitor should be reported to the FDA through MedWatch.
References:
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. NEJM 2017;377:644-657
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. NEJM 2015;373:2117-2128.
- Rawla P, Vellipuram AR, Bandaru SS, Raj JP. Euglycemic diabetic ketoacidosis: a diagnostic and therapeutic dilemma. Diabetes Care 2017;38:1638-1642.
- U.S. Food and Drug Administration. Drug Safety Communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood [Internet], 15 May 2015. Available from http://www.fda.gov/downloads/Drugs/DrugSafety/UCM446954.pdf. Accessed 24 June 2019
- Modi A, Agrawal A, Morgan F. Euglycemic diabetic ketoacidosis: a review. Current Diabetes Review 2017; 13:315-321.
- Yu Xiaofang, Zhang S, Zhang L. Newer perspectives of mechanisms for euglycemic diabetic ketoacidosis. International J of Endocrinology 2018;1-8.
- Peters AL, Buschur EO, Buse JB, et al. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015; 38:1687-1693.
- Munro JF, Campbell IW, McCuish AC, et al. Euglycaemic Diabetic Ketoacidosis. Br Med J 1973;578-580
- Karakaya Z, Topal FE, Topal F, et al. Euglisemic diabetic ketoacidotic coma caused by dapagliflozin. Am J of Emergency Medicine 2018:36;2136.e1-2136.e2.
- Dull RB, Spangler ML, Knezevich EL, Lau BM. Euglycemic diabetic ketoacidosis associated with sodium-glucose cotransporter type 2 inhibitors in patients with type 2 diabetes mellitus receiving oral therapy. J of Pharmacy Practice 2017;1-4.
- Andrews TJ, Cox RD, Parker C, Kolb J. Euglycemic diabetic ketoacidosis with elevated acetone in a patient taking a sodium-glucose cotransporter-2 (SGLT2) inhibitor. J of Emergency Medicine 2016;52:223-226.
- Lucero P, Chapela S. Euglycemic diabetic ketoacidosis in the ICU: 3 case reports and review of literature. Case Reports in Crit Care 2018;1-6.
- Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care 2015;38(9):1638-1642.